As of March 21st ,The Food and Drug Administration (FDA) has recently recalled three brands of eye drops, including one that has been linked to serious infections, vision loss and a death.
Recall eye drops refer to the act of removing a particular eye drop product from the market due to safety concerns or defects. This action is usually taken by the manufacturer or regulatory authorities to protect consumers from harm.
Patients are advised to stop using any of this brands:
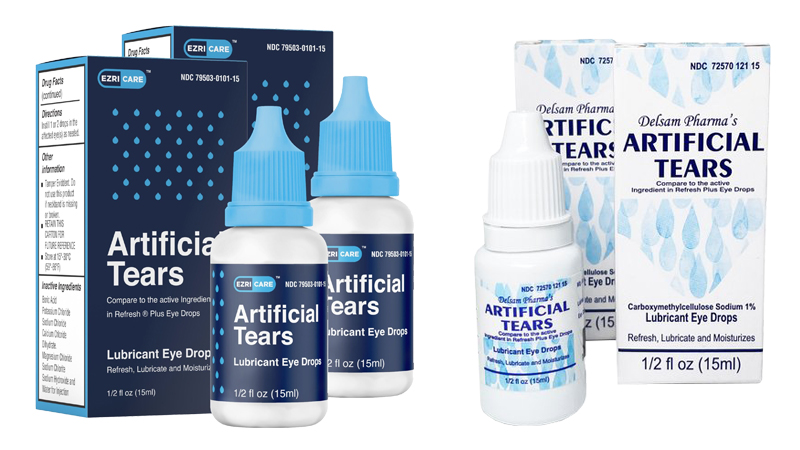
EzriCare Lubricant Eye Drops or Delsam Pharma’s Artificial Tears manufactured by EzriCare were associated with severe eye infection caused by a bacteria called Pseudomonas aeruginosa.
Purely Soothing 15% MSM Drops manufactured by Pharmedica USA was voluntarily recalling two lots of Purely Soothing Drops. This over-the-counter product is being recalled due to non-sterility. There have not been reports of illness or infection related to the product.
Brimonidine Tartrate Ophthalmic Solution, 0.15%. manufactured by Apotex Corp. initiated a voluntary recall for six lots of Brimonidine Tartrate Ophthalmic Solution on March 1 due to cracks in the caps. No infections or illness have been associated with this product.
Global Pharma Healthcare Artificial Eye Ointment due to possible bacterial contamination. No infections or illness have been associated with this product.
 Eye infection symptoms may include:
Eye infection symptoms may include:
- Yellow, green, or clear discharge from the eye
- Eye pain or discomfort
- Redness of the eye or eyelid
- Feeling of something in your eye (foreign body sensation)
- Increased sensitivity to light
- Blurry vision
At this time, there is no recommendation for testing of patients who have used any of these products and who are not experiencing any signs or symptoms of infection.
If you have any concerns about a specific eye drop product, it is recommended that you consult with your healthcare provider or pharmacist for guidance. They can advise you on whether the product has been recalled or if there are any safety concerns you should be aware of.